

22, 23, 24, 25 studied methane displacement and shale gas molecular dynamics simulation and obtained adsorption characteristics of the gas on graphite and carbon nanotubes, the law of entropy change and enthalpy change, and free energy change in the process of adsorption or displacement. 21 investigated the shale gas adsorption and diffusion in inorganic nanopores by molecular simulation. 20 also analyzed the CO formation and release rules in mines without spontaneous combustion, and conducted a targeted study on the influence of inertinite and vitrinite on CO adsorption in different coal ranks. 19 conducted experiments to study the generation and release laws of the original CO in coal seams and verified the existence of other CO sources besides spontaneous combustion. For the adsorption of CO on coal, related research is relatively scarce. 18 studied the adsorption characteristics of CO 2/CH 4/N 2/H 2O single-components and multi-components on lignite. 17 further studied the competitive adsorption characteristics of CO 2/CH 4/H 2O in different coal ranks.
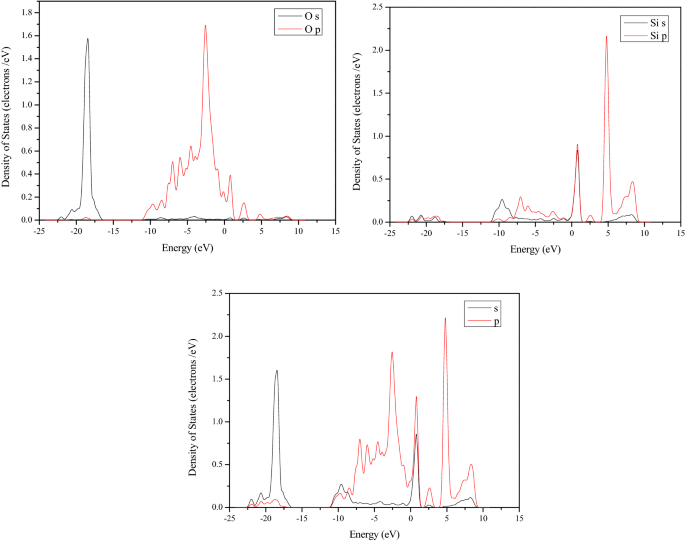
16 used molecular simulation methods to study the competitive adsorption characteristics of CO 2/CH 4/H 2O on lignite, while Yu et al. 15 used a Monte Carlo-based molecular simulation method to study the effect of moisture content on coal adsorption of methane. 13 studied the adsorption characteristics of coal to supercritical CO 2 and verified the adaptability of the Langmuir equation to the fitting of coal adsorption isotherms, and the adsorption form of supercritical CO 2 was analyzed. Many scholars have made studies on coal adsorption gases. The adsorption capacity of gas in coal is mainly affected by coal rank 6, pore structure 7, 8, pressure and temperature 9, moisture content 10, functional groups 11. Gases present in coal mainly enter through physical adsorption. Therefore, understanding the CO adsorption mechanism and the adsorption competitive relationship between CO, O 2, N 2, and CO 2 is of great significance to the control of CO in working faces. Coal is a porous material with surface area and pore volume, which provides conditions for gas adsorption. Affected by mining, part of residual broken coal is left in the goaf, and the CO can desorb under various conditions and spread to the working face, which brings great difficulties to coal mine work. The literature shows the CO comes from the coal formation stage in these mines, instead of oxidation or spontaneous combustion 5. In many low-rank coal mines, CO continues to exceed the standard and no signs of spontaneous combustion have been found. However, in recent years, it has been proved that spontaneous combustion of coal is not the only source of CO. Therefore, CO is widely used as an index gas to judge spontaneous combustion in many coal mines of China 4. For the generation of CO, it is generally considered that CO comes from the oxidation and spontaneous combustion of coal or mine explosion 2, 3, and no attention has been paid to the desorption of original CO in coal. Therefore, to prevent the desorption of the original CO in the goaf, it is not suitable to use CO 2 or N 2 injection for fire prevention, and the air leakage at the working faces need to be controlled.ĬO is a toxic and harmful gas in a coal mine, which affects the health of miners and restricts the production of coal mines 1. From the simulation, it can be seen that CO 2, N 2 or O 2 will occupy adsorption sites, causing CO desorption. In the low-pressure zone, the competitive adsorption capacity of CO 2 is stronger than that of CO, and the CO is stronger than N 2 or O 2. For binary-components, the competitive adsorption capacities of CO 2 and CO are approximate. The results show that for single-components, the order of adsorption capacity is CO 2 > CO > O 2 > N 2. Based on molecular dynamics, the Grand Canonical Monte Carlo method was used to simulate the adsorption characteristics and the Langmuir equation was used to fit the adsorption isotherms of gases.

To study the adsorption characteristics of CO, CO 2, N 2, O 2, and their binary-components in lignite coal, reveal the influence of CO 2 or N 2 injection and air leakage on the desorption of CO in goafs, a lignite model (C 206H 206N 2O 44) was established, and the supercell structure was optimized under temperatures of 288.15–318.15 K for molecular simulation.


 0 kommentar(er)
0 kommentar(er)
